KEYNOTE SPEAKERS

Nicola Bonucci, DEA, DESS, LLM
Director, Directorate for Legal Affairs, OECD, Paris, France

Charles Cain, Esq.
Deputy Chief, FCPA Unit, US Securities and Exchange Commission, Washington, DC

Heitor Costa
Executive Director, Portuguese Association of the Pharmaceutical Industry (APIFARMA), Lisbon, Portugal

Thomas Cueni
Director General, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland

Agustín Puente Escobar, Esq.
Head, Legal Cabinet, Spanish Agency for Data Protection, Madrid, Spain

Luca Genovese
Researcher, Access to Medicine Foundation, The Netherlands

Tarek Helou, Esq.
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, U.S. Department of Justice, Washington, DC, USA

Rui Santos Ivo
Vice president, INFARMED (Portugal National Authority on Medicines and Health Products), Member of the Management Board, European Medicines Agency (EMA), Lisbon, Portugal

George “Ren” McEachern,
CFE, CAMS
Supervisory Special Agent, International Corruption Squad, US Federal Bureau of Investigation, Washington, DC, USA

Marie-Claire Pickaert
Deputy Director General, Chief Financial Officer and Ambassador to the Medical Communities, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium

Aleksandar Rusanov, Esq.
Legal Advisor, Legal Department, European Medicines Agency (EMA), London, United Kingdom

Neill Stansbury, Esq.
Co-founder and Director of the Global Infrastructure Anti-Corruption Centre, Chair, International Organization for Standardization Anti-Bribery Project Committee, Vice Chair, Anti-corruption Standing Committee, World Federation of Engineering Organization, Buckinghamshire, UK
CO CHAIRS

Dante Beccaria
Global Compliance Officer and Vice President, Sanofi, Former Vice President of Internal Audit, Sanofi, Paris, France

Stephen Nguyen Duc
Former Office of Ethics & Compliance Area Director, International Operations, Western Europe & Canada, AbbVie, Board Member, Strategic Committee International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Rungis, France

Dominique Laymand, Esq.
Senior Vice President, Chief Ethics and Compliance Officer, Ipsen; President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France

Roeland Van Aelst
Regional Vice President – HCCO MD&D EMEA & Canada, Office Health Care Compliance and Privacy, Johnson & Johnson, Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Chairman, MedTech Compliance Network, Brussels, Belgium
FEATURING CHIEF COMPLIANCE OFFICERS

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma PLC, Former Vice President, Chief Compliance Officer, Dendreon, Former Associate General Counsel; Executive Director of Compliance, Seattle Genetics, Chicago, IL, USA

Sujata Dayal, JD
Vice President, Health Care Compliance and Privacy, Global Chief Compliance Officer, Pharmaceuticals, Johnson and Johnson, Former Chief Compliance Officer and Corporate Vice President, Biomet, Inc., Former Ethics and Compliance Officer, Abbott Laboratories, Titusville, NJ, USA

Martin Fuerle, MBA
General Counsel and Chief Compliance Officer, Vice President Risk & Compliance, Daiichi Sankyo Europe GmbH, Munich, Germany

Lori V. Queisser, CPA
Senior Vice President and Global Compliance Officer, Teva Pharmaceuticals, Philadelphia, PA, USA

Uffe Kåre Rasmussen, M.Sc.
Senior Director and Chief Compliance Officer, H. Lundbeck A/S, Former Principal Scientist, Novo Nordisk A/S, Copenhagen, Denmark
EFPIA CODES TRAINING WORKSHOP
The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA CODES TRAINING WORKSHOP on Monday, May 15, 2017.

FEATURED FACULTY

Ted Acosta, Esq.
Americas Vice Chair, Risk Management, EY; Former Senior Counsel, Office of Inspector General, United States Department of Health and Human Services, New York, NY, USA and Paris, France

David Andersen
Partner, Pharmaceuticals and Life Sciences, PwC, London, UK

Michael Bartke, PhD
Former Director Compliance Management, Daiichi Sankyo Europe GmbH, Strategic Committee, International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Munich, Germany

Ann Beasley, JD
Director, Healthcare and Life Sciences Disputes, Compliance & Investigations, Navigant Consulting, Former Chief Compliance Officer, Biogen, Former Global Compliance Officer, Novartis, Boston, MA, USA

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY, USA

Peter W.L. Bogaert, MA, LJ
Partner, Covington & Burling LLP, Brussels, Belgium

Rifat Bozacioglu
Area Compliance Director, Middle East, GlaxoSmithKline plc, Istanbul, Turkey

Chrissy Bradshaw, JD
Vice President, Porzio Life Sciences, LLC, Morristown, NJ, USA

Anne-Sophie Bricca
Deputy General Counsel, Senior Director Law and Compliance, EMEA, Terumo BCT, Brussels, Belgium

Anna Byrom, LLM
Associate Director, Compliance EUCA, Intercept Pharmaceuticals, Former Associate Director, Astellas Pharma Europe, London, United Kingdom

Jeffrey Campbell, JD
President and Chief Executive Officer, Porzio Life Sciences, Porzio Life Sciences, LLC, Morristown, NJ

Grant H. Castle, Esq.
Partner, Covington & Burling LLP, London, United Kingdom

Holger Diener, PhD
Managing Director, Association of Voluntary Self-Regulation for the Pharmaceutical Industry, Berlin, Germany

Peter Dieners, Esq.
Partner and Head, Global Healthcare and Life Sciences Group, Clifford Chance, Co-chair, Legal Affairs Focus Group (LA FG), EUCOMED, Co-chair, Compliance Network (CN), EUCOMED, Düsseldorf, Germany

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, United Kingdom

Karen Eryou
Head, Ethics & Compliance Programs, UCB Pharma, Former Co-chair, Asia Pacific Pharma Compliance Congress, Brussels, Belgium

George Fife
Partner, Fraud Investigation & Dispute Services, EY; Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France

Pedro Caridade de Freitas, LLM, PhD
Legal and Compliance Director, Portuguese Association of the Pharmaceutical Industry (APIFARMA), Lisbon, Portugal

Geert van Gansewinkel
Managing Partner, Europe and Asia Pacific, Polaris, Amsterdam, Netherlands

Dominik Geller
Head Pharma Healthcare Compliance Office, Roche, Basel, Switzerland

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA

Michael Alan Gleave
Compliance Officer, Corporate Compliance and CSR, H. Lundbeck A/S, Copenhagen, Denmark

Filipa Godinho
Business Consultant, Global Transparency, Eli Lilly and Company, Lisbon, Portugal

Ulf H. Grundmann, Esq.
Partner, Life Sciences Practice Group, King & Spalding LLP, Frankfurt am Main, Germany

Joe Henein, Pharm.D.
President and Chief Executive Officer, NewBridge Pharmaceuticals FZ LLC, Dubai, United Arab Emirates

Chris J Holmes
Director, Healthcare and Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant, London, United Kingdom

Casey Horton
Director, Healthcare and Life Sciences Disputes, Regulatory Compliance and Investigations, Navigant, Chicago, IL, USA

Bella Rafael Hovhannisyan
Compliance Director, Intercontinental Commercial Operations, CSL Behring, Former Compliance and Ethics Lead, Spain, Portugal, and Nordic Region, Bristol-Myers Squibb, Bern, Switzerland

Rajiv Joshi, ICAI, ISACA, CIA, CFE
Partner, Fraud Investigation & Dispute Services, EY, Mumbai, India

Carl Judge
Partner, Fraud Investigation & Dispute Services, EY, London, UK

Anita K. Kim-Reinartz
Partner, Fraud Investigation & Dispute Services, EY, Düsseldorf, Germany

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC

Kirtis Kraeuter, MGA
Head, Worldwide Compliance Committee and Monitoring Center of Expertise, Bristol-Myers Squibb, Princeton, NJ, USA

Edoardo Lazzarini, PhD
Compliance Initiatives Director EUCAN, Takeda Pharmaceuticals International, Former Compliance Officer EMEA (Europe, Middle East & Africa), Biomet, Milano, Italy

William Long, LLB
Partner, Sidley Austin LLP, London, United Kingdom

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting United States Attorney, District of Massachusetts, United States Department of Justice, Washington, DC

Abdul Luheshi, MBA
Executive Director, Global Operations, Ethics & Compliance, Astellas Pharma EMEA, Former Vice President, Health Care Compliance, Johnson & Johnson, Chertsey, UK

Marcel Maderitsch, LLM
Senior Compliance Officer, Vifor Pharma, Zürich, Switzerland

Maureen McGirr, JD
Vice President, Office of Ethics, Global Compliance Organization, Merck, Sharp, and Dohme, Kenilworth, NJ, USA

Sofie Melis
Senior Manager, Ethics and Compliance, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie – CIS, Limited, Moscow, Russia

Audrey Mills, JD
Sr. Director Ethics and Compliance and Chief Privacy Officer, Eli Lilly and Company, Indianapolis, IN, USA

Laura Nassar
Head of Compliance, Middle East, Roche Pharmaceuticals, Dubai, United Arab Emirates

Chrisoula Nikidis
Executive Director, Ethics and Compliance, Innovative Medicines Canada; Industry Co-Chair Designate, APEC Biopharmaceutical Working Group on Ethics, Ottawa, Canada

David O’Shaughnessy
Vice President, Compliance, Emerging Markets, Quintiles, Board Member, ETHICS, Former Vice President Compliance, International Pharmaceuticals, GSK, Former Vice President, Global Compliance Strategy, AstraZeneca, Reading, UK

Pascale Paimbault
Executive Director, EMEA Ethics and Compliance, ABAC, Astellas Pharma Europe; Former Compliance Officer, France -Benelux, Biogen, Treasurer and Strategic Committee Member, ETHICS; Former Chief Compliance Officer EMEA, Wright Medical Inc.; Former Senior Director, Compliance and Ethics, Business Program, EMEA, Bristol-Myers Squibb, Paris, France

Giota Papamarkou
Global Ethics & Compliance Director, Ipsen, Former EMEA Compliance and Ethics Manager, Bristol-Myers Squibb, Paris, France

Ima Parrondo
Health Care Compliance Officer EMEA-C, Janssen Pharmaceutical Companies of Johnson & Johnson, Madrid, Spain

Debolina Partap, BCOM, LLM
General Counsel, Wockhardt, Mumbai, India

Oscar Perdomo
Director, Pharmaceuticals and Life Sciences, PwC, Switzerland

Jessica Pfennig, EMBA, MLS
Global Commercial Operations, Global Digital Governance Director, AstraZeneca, Wilmington, Delaware, USA

Ariadna Quesada, MPP
Compliance Manager Europe, Middle-East and Africa, MicroPort Orthopedics Inc., Amsterdam, Netherlands

Jose Ramos, MD, MBA
Compliance Officer for Italy, Portugal, and Spain, Biogen, Former Ethics and Compliance Officer, Eli Lilly, Madrid, Spain

Vivian Robinson, Esq.
Queen’s Counsel, Partner, McGuire Woods, Former General Counsel of the UK Serious Fraud Office, Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Former Treasurer of Inner Temple, London, UK

Fabien Roy, DESS
Senior Associate, Hogan Lovells, Member, EFGCP-MedTech Europe Working Party, Brussels, Belgium

Dream Samir
Consultant, AMICULUM-ME, Secretary General, Pharmaceutical Research and Manufacturers Association, Gulf (PhRMAG),, Dubai, United Arab Emirates

Daniel Schafaghi
Global Compliance Operating Officer, Head of Compliance Emerging Markets, Boehringer Ingelheim GmbH, Ingelheim, Germany

Brian Sharkey, JD
Vice President, Porzio Life Sciences, LLC, Morristown, NJ, USA

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority, London, United Kingdom

Michele Tagliaferri, Esq.
Partner, Sidley Austin LLP, Brussels, Belgium

Melda Tanyeri, MS, MBA
Director, Fraud Investigation & Dispute Services, EY, London, UK

Yuet Ming Tham, JD
Partner, Sidley Austin LLP; Former Asia Pacific Regional Compliance, Director, Pfizer; Former Deputy Public Prosecutor, Singapore, Hong Kong

Tamara Tubin, lic. iur.
Compliance Director EUCAN, Takeda Pharmaceuticals International AG; Former Corporate Compliance Director, Biogen International; Former Ethics and Compliance Director International, Carefusion, Zurich, Switzerland

Ken Walsh, MA, MSc
Director, Life Sciences Consulting, Navigant, London, United Kingdom

Frank Wartenberg, PhD
President, Central Europe, IMS Health, Lecturer, Health Care Management Institute, EBS Business School, Frankfurt/Main, Germany

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland

Elisabethann Wright, Esq.
Partner, Hogan Lovells International LLP, Brussels, Belgium

Jolanta Wyszynska
Vice President, Compliance Europe, AstraZeneca, Warsaw, Poland

Maged Zaki, MD
Health Care Compliance Officer; Europe, Middle East and Africa, Johnson and Johnson Global R&D, Dubai, United Arab Emirates

Jose F. Zamarriego Izquierdo, PhD
Director Unidad de Supervision Deontologica, Farmainidustria, Madrid, Spain
2017-2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
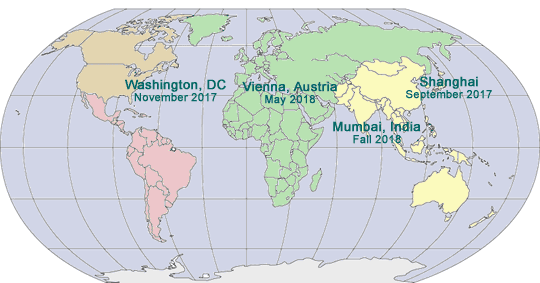
ELEVENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
May 15 – 17, 2017
Lisbon Marriott Hotel
Lisbon, Portugal
www.InternationalPharmaCongress.com
SEVENTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September 13 – 15, 2017
InterContinental Shanghai, Shanghai, China
www.AsianPharmaCongress.com
EIGHTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 6 – 8, 2017
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
INDIAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
International Society of Health-
care Compliance Professionals (ETHICS)
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Spring 2018
Mumbai, India
Coming Soon


